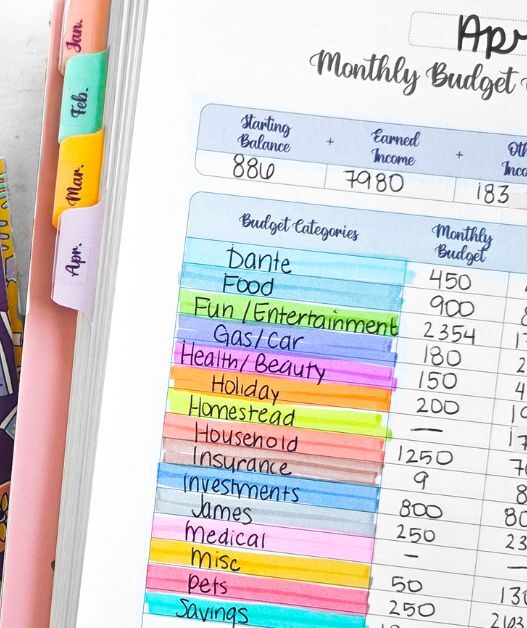
The Hidden Power of Paying Attention: Habit Stacking, Frugality, and Making the Most of What You've Got | brokeGIRLrich
Lately, I've been thinking about habit stacking and how it overlaps with frugality in some really practical ways. Not just in the “put your vitamins next to your toothbrush so you remember to take them” kind of way (though that works). But more in how little clusters of usefulness can form in our lives, if we're paying attention.
That's the real trick, I think: awareness.
Take my commute, for example. About six months after I moved to the far edge of nowhere, my school finally reached out, concerned about how far away I lived. Six months. Not a single missed class or appointment. I basically told them: too late. Because honestly, I've come to find that once-a-week commute incredibly productive.
I get on the train at the first stop, grab a table seat, and by the time I arrive in the city, I've done 80 minutes of reading or writing. It's time that could be “lost,” but I've trained myself to see it differently. And even on the mornings where I wake up thinking “absolutely not” (hello, February at 6 AM), once I'm up and at the station, the inertia kicks in. Might as well make something of the day. Even my backup plan, listening to care ethics or immersive theatre podcasts, turns the time into something useful.
It's not just about the train, either. If I'm heading into London anyway, why not stack something else on top? Immersive theatre performances, errands, even volunteering at a show I already wanted to see – all ways to maximize the value of a trip and cut down on extra travel costs. I've saved a good £50 just by being thoughtful about timing.
That's the kind of frugality no spreadsheet can teach you. It's the quiet kind, rooted in noticing patterns and opportunities:
- Are there sale cycles at your favorite shops?
- Do you have a system for actually using your loyalty cards?
- Is there a best day to travel, and can you plan around it?
- Do you keep an eye out for free events, or local experiences?
I realized recently that part of why I like wandering through shops when I'm not in a rush is that I'm scanning. Not just for things I need, but to keep a mental inventory of what's out there, how much things cost, where to find that weird prop if I ever need one (thanks, stage managing). It's casual research, but over time, it really adds up.











![Swagbucks Review [Is Swagbucks Legit?]](https://rjema.com/wp-content/uploads/2025/07/1753824180_Swagbucks-Review-Is-Swagbucks-Legit-527x630.jpg)